Tobacco and Cancer
The Environmental Protection Agency's (EPA) flawed study of environmental tobacco smoke and lung cancer.
Recently, the Environmental Protection Agency (EPA) completed a report concluding that exposure to environmental tobacco smoke (ETS) – the residual material from burning cigarettes that is released into indoor air environments by the process of active smoking – presents a serious and substantial public health problem. The EPA bases its conclusions not on any definitive set of data demonstrating causality, but on a generalized “total weight of evidence” that, in aggregate, implied causality to the EPA. In reaching those conclusions, the EPA ignored classic criteria for cause-and-effect relationships employed by the scientific community.
Without a clearly established mechanism for determining causality, declaring that a substance in our environment poses a significant health risk usually rests upon the convergence of three cornerstones of scientific evidence. These include: (1) evidence from population studies that exposure to the agent is associated with the development of disease in humans; (2) evidence that exposure to a specific substance or agent results in a specific disease; and (3) evidence that the specific agent causes the disease in question in a certain measurable dose (accumulative or otherwise) or at a certain level of exposure. For many potentially toxic environmental agents, the last two criteria are often, if not almost always, fulfilled through experimental animal studies. These criteria apply not only to carcinogens but, more generally, to any potentially toxic substance that causes any kind of disease.
The EPA’s conclusions regarding ETS, however, did not satisfy those evidentiary criteria. Instead, the EPA “weighted” selected data in an attempt to support its conclusions by other means. A critical assessment of the validity of the EPA’s conclusions, then, requires careful understanding of the manipulations by which evidence was weighted.
The EPA report is over 500 pages long and contains an unusually large amount of technical theory and background information. Comprehensively reviewing the report in its entirety is not possible in this relatively brief space. The purpose of this article is to address the more important parts of the EPA report that pertain to adults who are exposed to ETS. We will address other non-cancer respiratory illnesses in adults, as well as respiratory illnesses in children, elsewhere. For adult nonsmokers, the EPA concluded that “ETS is a human lung carcinogen, responsible for approximately 3,000 lung cancer deaths annually in U.S. nonsmokers.”
As a nation, we depend on the EPA to undertake risk assessments on many agents in our environment that might be potentially harmful to us. When the EPA “speaks,” enormous weight is given to its findings. We generally presume that its conclusions are based on solid scientific evidence and are derived by standard scientific practices.
Our presumption would be overgenerous in the case of the ETS report, unfortunately. In this case, the EPA’s risk assessment is built on the manipulation of data, ignores critical chemical analyses and key epidemiological data, violates time-honored statistical principles, fails to control adequately for important confounding influences (factors other than the one studied that may affect a result or a conclusion) that provide alternative explanations for its conclusions, and violates its own guidelines for assessing and establishing risk to a potential environmental toxin. It lacks credible quality control and adequate external unbiased peer review. In short, in its report on ETS, the EPA did not comply with accepted principles of toxicology, chemistry, and epidemiology, nor with its own guidelines for undertaking cancer risk assessment. In fact, the conclusions drawn by the EPA are not even supported by the EPA’s own statements.
In critically questioning these matters, however, we are not saying that exposure to ETS is without hazard. The data that have been presented in the literature, though, simply do not support any definitive conclusions. We believe that reasonable scientists could interpret the published literature on ETS with differing opinions. Nor are we suggesting that ETS should not be taken seriously. There are almost 50 million active smokers in the United States, and the better part of a billion smokers worldwide. Because of the large number of nonsmokers who are in contact with active smokers, concerns about any potential health risks associated with exposure to ETS are very important. It is an issue that deserves resolution by the highest quality of data that science has to offer, not by compromising well- established scientific principles or by distorting scientific fact.
In analyzing the EPA’s report, it is important to understand exactly what ETS is, and, perhaps more importantly, to understand what it is not. Some reports treat ETS as if it were a simple, discrete entity. Others consider ETS to be a collection of several individual or separate constituents, each of which can be quantified in a given sample of environmental air and assessed for risk separately. Still others have treated the different kinds of tobacco smoke, including ETS, as if they were all one and the same. Although giving some very limited passing acknowledgment to the actual nature of individual constituents in ETS, the EPA for the most part treated ETS as if it were a discrete entity with characteristics and health risks assumed comparable to the smoke that is inhaled from cigarettes by active smokers. In other words, the EPA based many of its conclusions on an explicitly stated assumption that because there is an association between active smoking and lung cancer there must also be a similar association between ETS and lung cancer.
The truth is that ETS is not a discrete entity; at least not one that can be completely measured or characterized as such under real-world conditions using currently available technology. The residual constituents of ETS change with time and differ in composition depending on the environment in which they are found. Concentrations of constituents also vary widely from time to time and from place to place. Furthermore, compared to other kinds of tobacco smoke, only a small fraction of the constituents of mainstream smoke and of sidestream smoke potentially present in ETS have ever been quantifiably identified in the real-world air to which the nonsmoker is exposed.
Sources of Environmental Tobacco Smoke
Not all tobacco smoke is the same. Three different types exist, all of which differ both physically and chemically. The first, mainstream tobacco smoke, is the material that is drawn through the butt, or mouth end, of the cigarette during active smoking; this is the tobacco smoke that smokers inhale into their lungs. Depending on how they inhale, and on whether or not they hold their breath, active smokers retain within their lungs somewhere in the range of 40 to 80 percent of the mainstream smoke that they generate. The remainder of the inhaled smoke that is not retained is exhaled as a potential contribution to ETS.
Mainstream tobacco smoke is complex. However, standardized and precise methods of reproducibly collecting and analyzing mainstream smoke have been established and accepted for years. There are over 5,000 well-characterized chemical components of mainstream smoke that by weight account for over 95 percent of the smoke. Some of these chemical components are recognized or designated human carcinogens; some are anti-carcinogens. Although several of the constituents of mainstream tobacco smoke have been considered, at one time or another, as the prime suspect allegedly responsible for causing lung cancer, no major carcinogen in smoke has ever been established. Indeed, the National Cancer Institute and other federal agencies spent hundreds of millions of dollars in a decade-long quest for a safe or less hazardous cigarette. In retrospect, that program could at best be considered cost-ineffective and at worst a failure. The specific carcinogenic needle in the burning haystack of cigarette smoke was never identified. It was hoped that if the cancer-causing factor in cigarettes could be identified clearly, it could be removed to make smoking less hazardous. This turned out to be an elusive goal.
One hundred thousand or more additional chemical components believed to be in mainstream smoke have not been well characterized and are present in only trace amounts. What goes into the respiratory system of the active smoker as mainstream smoke, however, is not what comes out as ETS. The inhaled mainstream smoke is stripped within the smoker’s respiratory system of many of its volatile chemical compounds. What are left then, as ETS, are small amounts of residual altered mainstream smoke particulates, saturated with water vapor by their passage through the respiratory system and dramatically reduced in volatile chemical constituents, as well as some gas phase residual constituents.
The second element of ETS, sidestream smoke, is the tobacco smoke that is released around the burning cone from the tip of the smoldering cigarette between active puffs. Sidestream smoke is also very complex. While smoldering, the cigarette burns at a lower temperature (500 to 600 degrees centigrade) than it does during the generation of an active puff (800 to 900 degrees centigrade). The chemical substances in sidestream smoke are similar to mainstream smoke, but the differences in temperature and burning characteristics cause significant differences in its chemical nature. Unlike mainstream smoke, standardized methods for collecting and assessing the chemical and physical characteristics of sidestream smoke do not exist. Regardless of those problems in measurement, sidestream smoke appears to be the major source (about 85 to 90 percent) of the residual tobacco smoke constituents that end up in ETS.
The third element of ETS is the very small amount of smoke that diffuses out of the cigarette through the wrapping paper, as well as the small amount of smoke that comes directly off of the burning cigarette tip during active puffing. For practical purposes, those contributions to ETS are negligible.
By weight, mainstream smoke is made up of about 70 percent air (drawn in through the cigarette) and about 10 or 11 percent water vapor. The remaining smoke is a complex mixture of thousands of chemicals. Many of the chemical constituents of tobacco smoke are highly reactive molecules that change within microseconds of their creation and are chemically unstable in our environment.
All forms of tobacco smoke have a certain density, defined as the concentration of particulates in the gas phase. If the particles are not dense enough to see, then the product usually is no longer defined as smoke. Compared to the other types of tobacco smoke, inhaled mainstream smoke is quite dense; exhaled mainstream smoke is diluted manyfold and usually is much less dense. Sidestream smoke starts out as being nearly equally dense near its point of emission, but as it moves even very small distances away from the burning cone it is diluted significantly.
ETS, on the other hand, is not dense at all; it is highly diluted. In fact, the residual constituents of mainstream and sidestream smoke that find their way into the air as ETS are so highly diluted that it is a misnomer to refer to them as smoke per se. The residual constituents present are diluted by a factor of thousands. But the EPA elected to equate ETS with mainstream and sidestream smoke as if they were all one and the same. The EPA simply chose not to address the fact that ETS has not been well characterized qualitatively or quantitatively; we do not even know exactly what is included in ETS.
In essence, the EPA assumed that if relatively large amounts of mainstream smoke are dangerous to active smokers, minuscule amounts will be hazardous to passive smokers. In some ways, that would not be an unreasonable approach if, indeed, nothing were known about the residual constituents of ETS. But something is known-namely, that certain residual constituents of tobacco smoke are sometimes present and sometimes not present, in infinitesimally small concentrations, in environmental air where active smoking is present. The assumption that all types of smoke are the same, however, is not supported by the available scientific data. This is extremely important, for one of the cardinal rules of environmental toxicology and risk assessment is to identify the specific chemical (or chemicals) of potential concern, because the biological responses to specific chemicals are in themselves highly specific.
The Nature of ETS
The EPA states quite authoritatively that “ETS is a complex mix of over 4,000 compounds.” The EPA states equally clearly that “this mix contains many known or suspected carcinogens or toxic agents.” Both statements are dubious. No scientific literature supports the assumption that ETS should be treated as a functional equivalent to mainstream smoke. Using the most sensitive of analytical detection systems, only small numbers of tobacco smoke residual constituents-in the range now of 50 to 100 or so-can be detected in environmental air under real-world circumstances, and then only at extremely low levels of concentration.
Over 5,000 compounds are identifiable in mainstream smoke collected under very carefully controlled circumstances immediately as it leaves the butt end of a cigarette. More than half that many compounds are identifiable in sidestream smoke collected as it leaves the burning tip of a cigarette. Is it reasonable to assume, as the EPA has apparently done, that all of those chemical compounds make their way into environmental air in a form that we as nonsmokers might passively inhale?
No, because as they age and become diluted in environmental air, some of the highly unstable residual constituents of tobacco smoke react chemically, adsorb onto surfaces in the environment, undergo a variety of other changes, or simply become so highly diluted that they have not been detected by known analytical means. Many of the chemical reactions are completed within microseconds.
The EPA report simply assumed that the potentially carcinogenic constituents in mainstream and sidestream smoke establish the carcinogenicity of ETS. There are, however, no data available in the EPA document or anywhere else to support that assumption. Independent studies on ETS have not indicated that it is a carcinogen. ETS is not mainstream smoke. ETS is not sidestream smoke. What nonsmokers might inhale passively in the presence of smokers is not quantitatively or qualitatively the same material that active smokers inhale from the butt end of a cigarette.
Dosimetry and Environmental Standards
An additional inviolable rule of environmental toxicology is that, in essence, “the dose makes the poison.” At some dose, every chemical is a potential poison. Some of our environmental chemicals are toxic to humans and about two dozen or so of them are designated as human carcinogens. But potential toxicity and carcinogenicity can be offset, for practical purposes, by limiting our exposure to acceptably low levels.
Levels of exposure to airborne environmental chemicals are usually expressed in terms of the amount of the chemical substance by its unit weight per volume of environmental air-for example, in milligrams (mg) of the specific chemical per cubic meter (m3) of air. For many chemicals, the acceptable level of exposure is often quite low, expressed as micrograms or nanograms per cubic meter of air. For comparative purposes, a milligram is one one-thousandth of a gram, a microgram (ug) is one one-millionth of a gram, and a nanogram (ng) is one one-billionth of a gram.
The simple exposure of humans to a given chemical, even if it is an established carcinogen, is by itself usually not associated with development of cancer. Essentially everyone in this country is exposed to potentially toxic or carcinogenic chemicals every day, but risk is not established by exposure alone. Rather, it is established through a dose-response relationship; accordingly, there is usually a specified level of exposure, or dose, that is accepted as at least relatively safe.
What do we really know about levels of exposure to ETS? The EPA report states, “Detailed chemical characterization of ETS emissions under conditions more typical of actual smoking conditions (e.g., using smokers rather than smoking machines) are limited.” Like the Emperor’s new clothes, not much is actually there. The report does list, but only in graphic form, six constituents of environmental air that are known residual environmental constituents of tobacco smoke, including formaldehyde, toluene, benzene, carbon monoxide, benzo[a]pyrene, and total polycyclic aromatic hydrocarbons. Limited attention is also given to two additional chemical constituents generally unique to tobacco-nicotine (and its metabolic breakdown product, cotinine) and the group of compounds known as tobacco-specific N-nitrosamines.
A recent scholarly monograph, published by Guerin, Jenkins, and Tomkins of the Oak Ridge National Laboratories, comprehensively reviewed several published sources from which a wide range of environmental levels of ETS constituents can be derived. That monograph is the source of the specific values for ETS environmental constituents cited here. The monograph is cited by the EPA report, but curiously enough, the data from it are never integrated into the assessment.
What, then, is the nature of the relative health hazards for the specific constituents of ETS listed by the EPA? One such constituent, formaldehyde, is designated as a potential carcinogen. Currently popular commercial cigarettes deliver about 20 to 90 micrograms of formaldehyde in mainstream smoke and up to 700 micrograms in sidestream smoke. Those numbers may seem high, but in comparison to other environmental sources they are not. Space heaters and gas ranges, for instance, release about 20,000 to 40,000 micrograms of formaldehyde per hour into our environment. Formaldehyde has also been used extensively in finishing and bonding wood products, and in coat fabrics and insulation products. In certain closed environments, such as a house trailer, formaldehyde can reach stable environmental concentrations in excess of 5,000 ug/m3. Formaldehyde also has been identified as one of the culprits in “sick building syndrome.”
In most buildings, however, the background levels of formaldehyde that we commonly are exposed to in everyday life are in the range of 40 to 50 ¬µg/m3. The best of the published data indicate that formaldehyde concentrations in ETS are similar to background levels and generally, with unusual exceptions, do not exceed 40 ¬µg/m3. The established “safe” level for environmental exposure to formaldehyde is 1,500 ¬µg/m3, or several fold the level attributable to ETS.
Benzene and toluene are also listed by the EPA as residual ETS constituents that are potential carcinogens. With high levels of exposure, they are associated in humans with the development of leukemia. With a limited number of exceptions, however, leukemia has not been consistently linked to active smoking, let alone exposure to the highly diluted concentrations of benzene and toluene that are present in ETS.
Benzene is ubiquitous in our environment, and toluene is chemically related to benzene. Gasoline is a primary source of benzene, toluene, and other related volatile organic chemicals (VOCs) in our air, as is outgassing from building materials, office activities and office machines, photocopying, various combustion sources, glue solvents, paint solvents, and the like. Frequently encountered background concentrations of VOCs in indoor air where residual constituents of ETS are expected to be found generally range from 2 to 20 Œºg/m3. The highest environmental concentrations of VOCs (100 Œºg/m3 and greater) are usually associated with sources other than ETS. Gasoline in the United States contains up to 2 percent benzene and filling one’s tank at a self-service gas station may result in higher levels of benzene exposure over a few minutes than would ever be encountered from ETS exposure for several hours. The established acceptable levels of exposure for benzene are 30,000 Œºg/m3 and for toluene are 375,000 Œºg/m3, values well above (over a thousandfold) any that might ever be expected from ETS.
Benzo[a]pyrene (BaP) is another aromatic hydrocarbon that has a high level of carcinogenicity for animals and is a suspected human carcinogen. Background indoor environmental concentrations of BaP generally range in the neighborhood of 0.1 to 1 ng/m3 without smokers present, and in the range of 0.3 to 1.5 ng/m3 with them. By comparison, outdoor levels of BaP in heavy traffic in urban areas or in areas close to industrial sources are in the 1 to 3 ng/m3 range. Some highly urbanized areas have shown polycyclic aromatic hydrocarbons (PAH) peak levels of 15-50 ng/m3. Standardized safe exposure levels for BaP have not been established. Our primary exposure to PAHs, however, comes not from our environmental air but from the food we eat and from the water we drink. Our dietary intake of BaP, for instance, is probably about 1,000 to 5,000 ng/day, without any charcoal- broiled meat. Drinking water contains 1 to 10 ng/L of PAHs and surface waters contain several hundred nanograms per liter. One piece of charcoal-broiled meat delivers about 2,000 to 3,000 ng of PAH. Surprisingly, however, probably the sources with the highest PAH levels in our diet are the leafy vegetables (e.g. lettuce, spinach, and unrefined grains), which are contaminated by outdoor deposition from the air.
Nicotine is more or less unique to tobacco, although very small amounts can be found in certain foodstuffs, such as tomatoes. Nicotine, however, has never been seriously considered a carcinogen. Some nitrosamines are also unique to tobacco. Nitrosamines are a suspected human carcinogen, based on animal studies, but their specific role in human carcinogenesis has remained controversial. Exposure to ETS residual constituents may, under some circumstances, result in the intake of 0.1 micrograms or less of nitrosamines per day by nonsmokers, a relatively minuscule amount compared to the 10 to 100 micrograms of nitrosamines ingested from food in the average diet each day.
Like the 50 to 100 other chemical compounds that are reported to have been measured in ETS, the constituents of ETS that are cited by the EPA are present only at infinitesimally low concentrations in our environment. If any of those constituents are, in fact, carcinogenic to humans at such very low levels, and if they are indeed present in our environment from ETS in concentrations that represent a true health hazard, those who are not smokers deserve to know that and to have a proper, credible risk assessment undertaken that is based on facts and reality, not on tiers of assumptions and extrapolations.
Assessing Health Risks
Because ETS has not been well characterized as a physical or chemical substance, and because the level of exposure to most of the residual constituents of tobacco smoke in environmental air is too low to be quantified under real-world conditions, assessing the purported health risks of passive smoking becomes very difficult. Two of the three cornerstones for determining a causal relationship-(1) establishing a specific substance that causes a specific disease and (2) establishing a dose relationship for the development of that disease-cannot be established on the basis of the data now available. The third remaining approach is to evaluate the potential health risks for nonsmokers in epidemiological studies.
Epidemiology studies employ statistical analyses to determine the rate and distribution of a disease (or diseases) within given human populations and, when possible, the factors that are associated with the development of that disease. Epidemiology studies are most effective when they can assess a specifically defined risk factor. Because exposure to residual constituents of tobacco smoke in our environment cannot be quantified, epidemiologists have again had to use indirect measurements, or proxies, of ETS exposure.
In its epidemiological risk assessments, the EPA employed previously published studies that evaluated the development, or lack of development, of lung cancer as a function of spousal smoking habits. These studies were based on a concept of “relative risk,” usually expressed as an odds ratio. Relative risk expresses statistical correlations for the rates of disease development in two populations; it is defined as the relationship of the rate of the development of a disease (in this instance, lung cancer) within a group of individuals (primarily nonsmoking wives) exposed to a variable in the population (spousal or husband’s smoking habits), divided by the rate of the same disease in individuals not exposed to this variable (lung cancer in nonsmokers married to nonsmokers). The resultant risk ratio or odds ratio is the calculated rate of disease studied in the exposed population divided by the rate of that disease in the unexposed population, as follows:
| Relative Risk = | Rate of lung cancer in nonsmoking women married to husbands who smoke _________________________________________ Rate of lung cancer in nonsmoking women married to husbands who do not smoke |
The terms “risk ratio,” “odds ratio,” and “relative risk” are often used interchangeably, especially for rare diseases like lung cancer. If the disease rates in the two populations studied (nonsmoking women married to smokers versus nonsmoking women married to nonsmokers) were exactly the same, the odds ratio or relative risk would be 1.0. If more lung cancers occurred in nonsmoking women married to smoking spouses than occurred in nonsmoking women married to nonsmoking spouses, the relative risk would be greater than 1.0.
Currently, there are at least 32 published studies in the literature that evaluate rates of lung cancer in women as a function of their husband’s smoking habits. The first of those studies was published in 1981 and the last two studies were published in 1992. Thirteen of the studies were conducted in the United States, and 19 have come from abroad. Most evaluate rates of lung cancer in nonsmoking females married to smoking males; one study evaluates data on a mixed male and female population; a few contain limited data on nonsmoking males married to smoking females. (Those limited data, although mentioned in passing, were not included in the EPA’s final analysis of lung cancer risk assessment.)
All of the studies assume, without measuring or attempting to quantify, that nonsmoking wives are exposed passively to the residual constituents of ETS generated within the home and elsewhere by their smoking spouses. The studies generally were based on questionnaire responses; actual levels of exposure to ETS constituents were not determined. Such questionnaires remain the only data available to assess the specific potential health effects of ETS on nonsmokers.
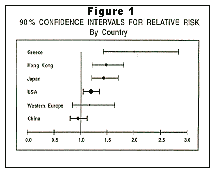
Outcome measures for studies conducted in various parts of the world varied considerably (See Figure 1, above). The reasons are not entirely clear, but it is presumed that other lifestyle factors (air pollution exposure, diet, cooking practices, racial genetic variation, etc.) are important variables that influence the development of lung cancer.
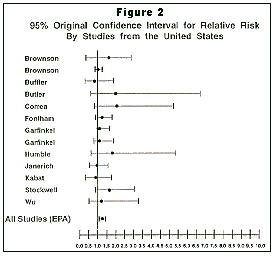
Although it tabulated summary data from all studies worldwide, the EPA based its risk assessment for lung cancer on only 11 of the 13 available studies from the United States. Because of the social, cultural, and racial differences that exist between widely diverse geographical areas, relying only on U.S. studies was a reasonable approach. The EPA chose to exclude the two most recent U.S. studies, however, simply because they were published after an arbitrary cut-off date earlier in 1992. Interestingly, one of the excluded studies, by Stockwell et al. from the National Cancer Institute, stated that for lung cancer “we found no statistically significant increase in risk associated with exposure to environmental tobacco smoke at work or during social activities.”
The odds ratio data from all 13 U.S. studies are presented in Table 1, above, and are expressed as the estimated value of relative risk. The EPA “adjusted” the originally published data, in theory correcting for potential misclassification of smokers as nonsmokers and other factors. Those “adjustments” were undertaken because questionnaires regarding smoking habits are notoriously limited and often inaccurate, largely because smoking has become a social taboo in this country, and active smokers sometimes deny their smoking practices when answering questionnaires. For their calculations, the EPA also selected “subsets” of data from the initially reported total data published. Table 1 provides both the EPA-adjusted data (sometimes representing only subsets of selected data) and the original data from the original publications.
The Magnitude of the Risk
A relative risk, or odds ratio, is characterized as strong or weak depending on its magnitude, or degree of association. A strong relative risk has an odds ratio of 5.0 to 10.0 or greater. By conventional definition, weak relative risks are ones where the odds ratio is in the range of 1.0 to 3.0 or so. In both the original data and in the EPA-adjusted data, all of the odds ratios are relatively small or weak. Three of the studies have an odds ratio of less than 1.0 (potentially suggesting less lung cancer occurs in nonsmokers married to smokers than occur in nonsmokers married to nonsmokers), and none of the studies report a strong relative risk.
 Could one conclude from these data that 10 of the studies demonstrate a small increased relative risk for the development of lung cancer on the basis of history of spousal smoking and three of the studies demonstrate a “protective” effect on the same basis? To answer that question, scientists, including epidemiologists, rely on measurement of what is termed “statistical significance,” which pertains to whether the observed result is related to the variable studied (in this instance, a smoking spouse) and not due to random variation or mere chance.
Could one conclude from these data that 10 of the studies demonstrate a small increased relative risk for the development of lung cancer on the basis of history of spousal smoking and three of the studies demonstrate a “protective” effect on the same basis? To answer that question, scientists, including epidemiologists, rely on measurement of what is termed “statistical significance,” which pertains to whether the observed result is related to the variable studied (in this instance, a smoking spouse) and not due to random variation or mere chance.
Science has established rules for determining statistical significance. With rare exceptions, scientific convention has established that something is probably “true” if there is no more than a 5 percent chance that the result could be attributed to mere chance. One commonly used statistical assessment of this measurement of random chance is the confidence interval.
A confidence interval is a numerical range of values that has a specified probability of including the true value (as opposed to the estimated value) within that range. A 95 percent confidence interval indicates that there is a 95 percent possibility that the observed result did not happen by chance and a 5 percent possibility that the observed result was due to chance alone. Using the Buffler study as one example from Table 1 the relative risk for developing lung cancer in a nonsmoker living with a spousal smoker was by the originally published calculation 0.80. If this average value were taken alone without some associated statistical test a “”protective”” effect would be implied based on the average odds ratio. The confidence interval for this study (at the 95 percent confidence level) however was 0.34 to 1.81. In other words with 95 percent confidence the real effect or true value of relative risk for this study was any odds ratio within the range of 0.34 to 1.81 although the distribution was weighted around an average of 0.80. Interpreted in that way the results have about as much chance of showing an increased risk as they do of showing decreased risk for developing lung cancer as a function of spousal smoking history. The odds ratio values in all of the ETS studies however are so small that any other minor factor could disturb the result.
Any odds ratio result whose range of confidence values reaches or passes through unity or 1.0 (the value of zero increased risk) is considered by conventional scientific rules to be statistically not significant. For a relative risk to be significant the range of values of the confidence interval must be entirely greater than or less than a reference value of 1.0. The odds ratios and their inclusive confidence intervals for all of the ETS lung cancer studies from the United States are shown graphically in Figure 2 above. Using the original results reported for the 13 studies from the United States all 13 studies failed to demonstrate a statistically significant relationship between spousal smoking and lung cancer in nonsmokers. Using the EPA-adjusted data 10 of the 11 studies employed in the EPA analysis also are unable to show a statistically significant risk.
When a series of epidemiologic data suggest an effect that sometimes reaches statistical significance and sometimes does not it may prove of value to combine all of the data from all of the studies into one comprehensive analysis. That pooling of data is called a “”meta-analysis.”” The EPA pooled the adjusted results of 11 studies into such a meta- analysis. The resultant relative risk or odds ratio for all of these studies’ combined values was 1.19 with a 90 percent confidence interval of 1.04 to 1.35. On the basis of the combined pooling of data or meta-analysis the EPA concluded that there was a 19 percent increased chance of developing lung cancer if you were a nonsmoker married to a smoking spouse although 10 of 11 studies from which the data were derived revealed no statistically significant effect even after being adjusted by the EPA.
Manipulation of Data
Is the EPA meta-analysis a scientifically valid manipulation of data? Combining data and undertaking a meta-analysis are valid procedures under appropriate circumstances. But in order to make the outcome value of their meta-analysis “”valid”” and “”statistically significant the EPA first had to adjust the data as originally published in peer-reviewed literature and second they had to broaden the confidence intervals to a scientifically unconventional level of 90 percent.
When a number of studies are combined the confidence intervals generally are “”ratcheted down or tightened to assess significance; the EPA did just the opposite and in so doing diminished its report’s scientific value. Lowering of statistical standards to make valid otherwise unmeaningful results is an unusual and dubious scientific practice. In the past the EPA has employed 95 percent confidence levels as a measure of scientific validity. Had the EPA done so in this case or had it not adjusted the original data its analysis would not have had the same outcome. If the EPA had included all of the available published data and not just 11 of 13 studies its outcome assessment also would have been different. The manipulation of data in this manner to develop statistical significance permitted the EPA to declare passive smoking a Group A carcinogen – the highest rank possible. Without the recalculations and manipulations the EPA would have not met any of the three classic criteria for establishing risk.
A relative risk of 1.19 even if the data were not manipulated is extremely weak. It is of the same general magnitude as the risk that an American citizen faces of dying in a bicycling accident over the course of a lifetime. It is a risk that is less than that associated with developing colon cancer by drinking chlorinated water which is in most U.S. cities’ water supplies. It is generally accepted in the medical literature that any time a relative risk is less than 2.0 the distinct possibility exists that the finding is artifactual and a consequence of the influence of confounding factors.
For instance many studies indicate that dietary factors alone can influence the rate of development of lung cancer both in smokers and in nonsmokers through a relative risk in the range of 20 to 30 percent or so the same relative magnitude of risk attributed to ETS by the EPA. Multiple reports from the National Cancer Institute and others demonstrate that because of their lifestyles the diets of smokers tend to be deficient in beta carotene vitamins A C and E folate selenium and other nutrients known to be anti-carcinogenic. In addition smokers have lower blood levels of beta carotene and other nutrients than can be explained by diet alone. Characteristically smokers exhibit other high-risk behaviors that reflect an unhealthy lifestyle. Although the degree to which nonsmoking spouses share such high-risk behaviors has not been extensively quantified and is currently under study it is only common sense that many of the various risks especially the dietary ones might be shared.
As individuals grow older they have an increased risk for the development of lung cancer as well as other cancers. Age then becomes a very important confounding variable in any study that evaluates the effect of an environmental agent on the development of lung cancer. The EPA analysis as well as some of the original reports did not control for this important variable.
There are more than 20 other confounding factors that have been identified as important to assessing risk for lung cancer. When the suggested relative risk is very low as it is in passive smoking a single uncontrolled or unaccounted variable can cause a totally spurious interpretation. The EPA’s risk assessment acknowledged that confounders are important to any evaluation of ETS as a potential carcinogen. Its concern for confounders was extremely limited however and their influence was evaluated by employing a modeling of data by a method as yet untested and unproved by conventional peer review. The EPA in essence ignored its own guidelines and established requirements to rule out confounding as an alternative explanation for an association before basing causal inference on epidemiologic results. Until studies take these variables into consideration we will never know the true risks of ETS exposure.
Safeguarding the Future
The EPA apparently at its own request recently underwent a review to identify how it could better use sound science as a foundation for its policy decisions. That review published as “”Safeguarding the Future: Credible Science Credible Decisions was critical of the EPA and included a set of guidelines to improve the quality of science in its risk assessments.
With its document on passive smoking the EPA disregarded the suggestions of its own review. Scientific integrity was compromised if not outright abused by the manner in which this risk assessment was generated. Abusing scientific integrity and generating faulty “”scientific”” outcomes through manipulations assumptions and extrapolations leads to the development of mistaken programs at enormous cost to our government and to taxpayers. Indeed the cost to the scientific process itself is even greater. Science should dictate what policies need to be established; predetermined policies should not dictate how science should be interpreted. We have many problems in the environment some of which are of far greater biological impact than our potential exposure to the residual constituents of ETS. The EPA is charged with addressing those problems critically objectively and honestly. Compromising the credibility of the EPA by adjusting science leaves us with an important resource substantially diminished. We need and we deserve better. Will reality and fact ever catch up with political science at the EPA?
Regulation: The Cato Review of Business & Government (1993 #3)
Gary L. Huber is a professor of medicine at University of Texas Health Center in Tyler, Texas. Robert E. Brockie is at the Presbyterian and Doctors Hospital in Dallas, Texas. Vijay K. Mahajan is a professor of medicine at St. Vincent’s Hospital in Toledo, Ohio.
![[EXBTA] Bundle: Tumor and Autoimmune](/web/image/product.template/2411/image_1920/%5BEXBTA%5D%20Bundle:%20Tumor%20and%20Autoimmune?unique=ae82545)
![[RLEL] Ellagica](/web/image/product.template/3145/image_1920/%5BRLEL%5D%20Ellagica?unique=ae82545)