Modified Citrus Pectin and Cancer
Pectin is a complex carbohydrate (polysaccharide) found in virtually all plants. Pectin helps to bind cells together and provides a structural framework for maintaining the shape and integrity of cell membranes. Recently, a modified form of citrus pectin derived from the pulp and peel of citrus fruits has been shown to attach to cancer cells to prevent them from spreading throughout the body, pointing the way to a potentially safe approach for preventing or reducing cancer metastases.
Spreading Metastases
Conventional cancer treatment involves surgery to remove primary tumors, followed by chemotherapy, radiation, or a combination of treatments designed to eradicate all remaining traces of cancer. This follow-up therapy is critical for addressing the biggest threat from cancer – the formation of secondary cancers, or metastases. Metastases are not new or different cancers, but new cancer colonies started from cells that have migrated to new sites. Sites where metastases commonly occur include the bones, lungs, prostate, kidney, liver, thyroid and brain. Left unchecked, metastases can quickly overwhelm the body's defenses. In fact, it is metastases, not primary tumors that are responsible for most cancer deaths.
Halting Metastases
Over the last two decades, research into controlling or halting cancer metastases has led to two promising new strategies. The first, antiangiogenesis, targets the growth of new blood vessels (angiogenesis) that are required for tumor growth. Originally pioneered by noted cancer researcher Dr. Judah Folkman, antiangiogenesis grew from his observation that tumors cannot grow without access to a constant supply of new blood vessels. Folkman theorized that cancer cells actively communicate with surrounding tissues to trigger the growth of new blood vessels (neovascularization) needed to supply nutrients and remove waste products.
Once neovascularization is initiated, hundreds of new capillaries converge on the tumor site and are quickly coated with new layers of rapidly dividing tumor cells. Folkman also theorized that, just as certain chemical messengers can initiate new capillary formation, other signals could inhibit neovascularization. This insight led to the development of antiangiogenic therapy, which, in contrast to other cancer treatments, doesn't directly destroy tumors, but aims to limit their blood supply, causing tumors to shrink.
By 1997 researchers were excited by promising results from several antiangiogenic drugs. Speaking of one early angiogenesis inhibitor called TNP470, in 1997 Folkman commented on the results of early clinical human trials, stating, “We've not seen a tumor that we cannot regress (shrink).” Currently TNP470 and several other angiogenesis inhibitors are in clinical trials, and other promising compounds are under study in university laboratories and in some 30 pharmaceutical and biotechnology companies around the world. For more information on nutritional compounds that have been shown to help inhibit new capillary growth and reduce angiogenesis refer to “Nutritional Support for Cellular Mutagenic Concerns.
Intercepting Cancer Cells
The second strategy for controlling metastases works by intercepting migrating cancer cells before they have a chance to establish new tumors. This approach targets a family of carbohydrate-binding proteins called lectins. Lectins are attracted to sugar molecules found on the surface of almost all cells. Lectins help cancer cells stick together to form multi-celled clusters that are believed to be necessary for metastases formation. Lectins also enable cancer cells to communicate with each other, as well as with other types of cells (cell-to-cell communication) to trigger cellular transformations that assist the spread of cancer.
One class of lectin – called galectins (for galactoside-binding lectins) – possesses an especially strong affinity for galactose, a simple sugar located on the surface of cells lining blood vessels. A number of cancer researchers have focused on galectin-3 that has been found to be directly involved in the progression and spread of several types of cancers, including breast, prostate and colon cancer. Serum levels of galectin-3 correlate closely with the spread of cancer, and may serve as a biological marker to help physicians and patients monitor the efficacy of anti-cancer therapies.
The Protein-Sugar Connection
The powerful attraction between galectins and galactose plays a pivotal role in how cancers spread in the body. After a cancer cell has broken free from its primary tumor (or is accidentally dislodged during surgery) it floats freely through the blood and lymph systems until it eventually becomes trapped in a small blood vessel (microcapillary). Firmly lodged in the microcapillary, galectins on the surface of the cancer cell start to bind to galactose receptors on endothelial cells (the cells that form the inside lining of blood vessels). After securely attaching to the endothelium the cancer cells penetrate through the blood vessel walls. The final step after invading the vessel involves the release of chemical signals that trigger new blood vessel growth (angiogenesis), and a new tumor colony is firmly established.
Modified Citrus Pectin
Modified citrus pectin (MCP) is a unique dietary fiber that is produced by processing natural citrus pectin by altering its pH and splitting the carbohydrate chains to form a low molecular-weight, water-soluble fiber that is rich in the sugar, galactose. It is this presence of particularly high amounts of galactose that led researchers to wonder if MCP might bind with proteins (lectins) on cancer cells to inhibit their ability to bind with other tissues.
Early test tube studies revealed that MCP did indeed bind to galectins from numerous human cancer cell lines to inhibit their ability to adhere to other cells. Researchers found that as little as a 1.0 percent solution of MCP inhibited attachment of 1) human prostate adenocarcinoma cells, 2) human breast carcinoma cells, 3) human melanoma cells, and 4) human laryngeal epidermoid carcinoma cells to human endothelial cells. (Naik H, et al.)
In 1992, Platt and colleagues demonstrated that MCP was effective at reducing metastases in mice injected with live melanoma cells. One group of mice was injected with normal melanoma cells, while a second group received melanoma cells that had been incubated in a solution containing MCP. Seventeen days after being injected, the mice receiving untreated melanoma cells were found to have, on average, 33 new tumors (metastases) in their lungs, while the mice receiving the MCP-treated cells had virtually no lung tumors. The researchers hypothesized that MCP had successfully attached to the lectin sites on the cancer cells, blocking the receptors and rendering them incapable of attaching to other cells.
In a second study conducted in 1995, Pienta and colleagues demonstrated that adding MCP to drinking water was an effective delivery route for reducing experimental metastases in rats. Four days after injecting rats with live prostate cancer cells, the animals were divided into three groups. Two groups of rats were treated with MCP added to their drinking water in amounts of 0.1% and 1.0%. The animals in the third group, the control, received no MCP.
Thirty days after being injected with one million active prostate cancer cells, 15 out of 16 rats in the control (untreated) group had cancer metastases in their lungs, compared with 7 of 14 rats in the 0.1% group, and 9 of 16 in the 1.0% group. Importantly, the 1.0% group had, on average, only one tumor per animal, versus an average of nine tumors in the lungs of the control group. Commenting on the results of the study the researchers noted that oral intake of modified citrus pectin acts as a “potent inhibitor of spontaneous prostate cancer metastasis…”
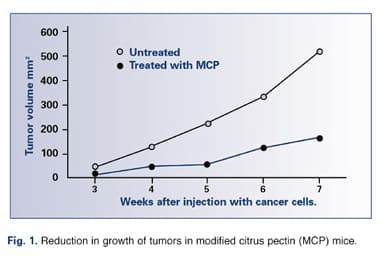
MCP Exhibits Antiangiogenesis Activity
A recent paper published in the December 2002 issue of the Journal of the National Cancer Institute found, as with earlier studies, that MCP significantly reduced both the incidence and the size of tumors in rats injected with human breast cancer and colon cancer cells. Additionally, in vitro experiments demonstrated that MCP inhibited formation of capillaries, demonstrating that MCP possesses antiangiogenic properties. Of their findings, the researchers concluded that, “MCP, given orally, inhibits carbohydrate-mediated tumor growth, angiogenesis, and metastasis in vivo, presumably via its effect on galectin-3 function. These data stress the importance of dietary carbohydrate compounds as agents for the prevention and/or treatment of cancer.
Conclusion and Dosage Recommendations
There are unfortunately no clinical studies that we are aware of to confirm the efficacy of Modified Citrus Pectin as an anti-cancer substance in humans. Nevertheless, we believe that because of its absolute lack of toxicity in any amount, its demonstrated efficacy in reducing the incidence and size of tumors in experimental animals, and its potential anti-cancer mechanisms as demonstrated in a number of in vitro models, Modified Citrus Pectin should be considered as a key part in any preventive or therapeutic regimen for any type of cancer. Dosage is also speculative, but based on the animal studies, we believe that a dosage of five grams per day may provide significant preventive or therapeutic benefits.
References
1. Al-Mehdi AB, et al. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat Med 2000;6:100-2.
2. Beuth J, et al.. Inhibition of liver tumor cell colonization in two animal tumor models by lectin blocking with D-galactose or arabinogalactan. Clin Exp Metastasis 1988;6:115-20.
3. Hartwell DW, Butterfield CE, Frenette PS, Kenyon BM, Hynes RO, Folkman J, et al. Angiogenesis in P- and E-selectin-deficient mice. Microcirculation 1998;5:173-8.
4. Hayashi A, Gillen AC, Lott JR. Effects of daily oral administration of quercetin chalcone and modified citrus pectin. Altern Med Rev 2000;5:546-52.
5. Hensel A, Meier K. Pectins and xyloglucans exhibit antimutagenic activities against nitroaromatic compounds. Planta Med 1999;65:395-9.
6. Hsieh TC, Wu JM. Biochem Mol Biol Int 1995;37:833-41.
7. Inohara H, Raz A. Effects of natural complex carbohydrate (citrus pectin) on murine melanoma cell properties related to galectin-3 functions. Glycoconj J 1994;11:527-32.
8. Inufusa H, Nakamura M, Adachi T, Aga M, Kurimoto M, Nakatani Y, et al. Role of galectin-3 in adenocarcinoma liver metastasis. Int J Oncol 2001;19:913-9.
9. Meromsky L, Lotan R, Raz A. Cancer Res 1986;46:5270-5.
10. Naik H, et al. Proc Annu Meet Am Assoc Cancer Res; 36:A377 1995.)
11. Nangia-Makker P, Baccarini S, Raz A. Carbohydrate-recognition and angiogenesis. Cancer Metastasis Rev 2000;19:51-7.
12. Nangia-Makker P, et al. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol 2000;156:899-909.
13. Nangia-Makker P, et al. The role of galectin-3 in tumor metastasis. In: Lectins and pathology. London (U.K.): Taylor & Francis, Inc.; 2000.
14. Pienta KJ, Naik H, Akhtar A, Yamazaki K, Replogle TS, Lehr J, et al. Inhibition of spontaneous metastasis in a rat prostate cancer model by oral administration of modified citrus pectin. J Natl Cancer Inst 1995;87:348-53.
15. Platt D, Raz A. Modulation of the lung colonization of B16-F1 melanoma cells by citrus pectin. J Natl Cancer Inst 1992;84:438-42.
16. Pratima Nangia-Makker, Victor Hogan, Yuichiro Honjo, Sara Baccarini, Larry Tait, Robert Bresalier, Avraham Raz. Inhibition of Human Cancer Cell Growth and Metastasis in Nude Mice by Oral Intake of Modified Citrus Pectin. J Natl Cancer Inst, Vol. 94, No. 24, December 18, 2002
17. Raz, A., & Lotan, R. “Endogenous galactoside-binding lectins: A new class of functional tumor cell surface molecules related to metastasis,” Cancer Metastasis Rev, 6: 433-52, 1987.
18. Smith-Barbaro P, Hanson D, Reddy BS. Carcinogen binding to various types of dietary fiber. J Natl Cancer Inst 1981;67:495-7.
19. Taper HS, Delzenne NM, Roberfroid MB. Growth inhibition of transplantable mouse tumors by non-digestible carbohydrates. Int J Cancer 1997;71:1109-12.
20. Van den Brule FA, Castronovo V. Laminin binding lectins during invasion and metastasis. In: Lectins and pathology. London (U.K.): Taylor & Francis, Inc.; 2000.
from Nutrition Review
![[EXBTA] Bundle: Tumor and Autoimmune](/web/image/product.template/2411/image_1920/%5BEXBTA%5D%20Bundle:%20Tumor%20and%20Autoimmune?unique=ae82545)
![[RLEL] Ellagica](/web/image/product.template/3145/image_1920/%5BRLEL%5D%20Ellagica?unique=ae82545)